May 1, 2021 to February 28, 2023 – pilot projects for Dietary supplements serialization
 Dietary supplements
Dietary supplements
Proposed architecture of Russian Track & Trace regulations for dietary supplements*
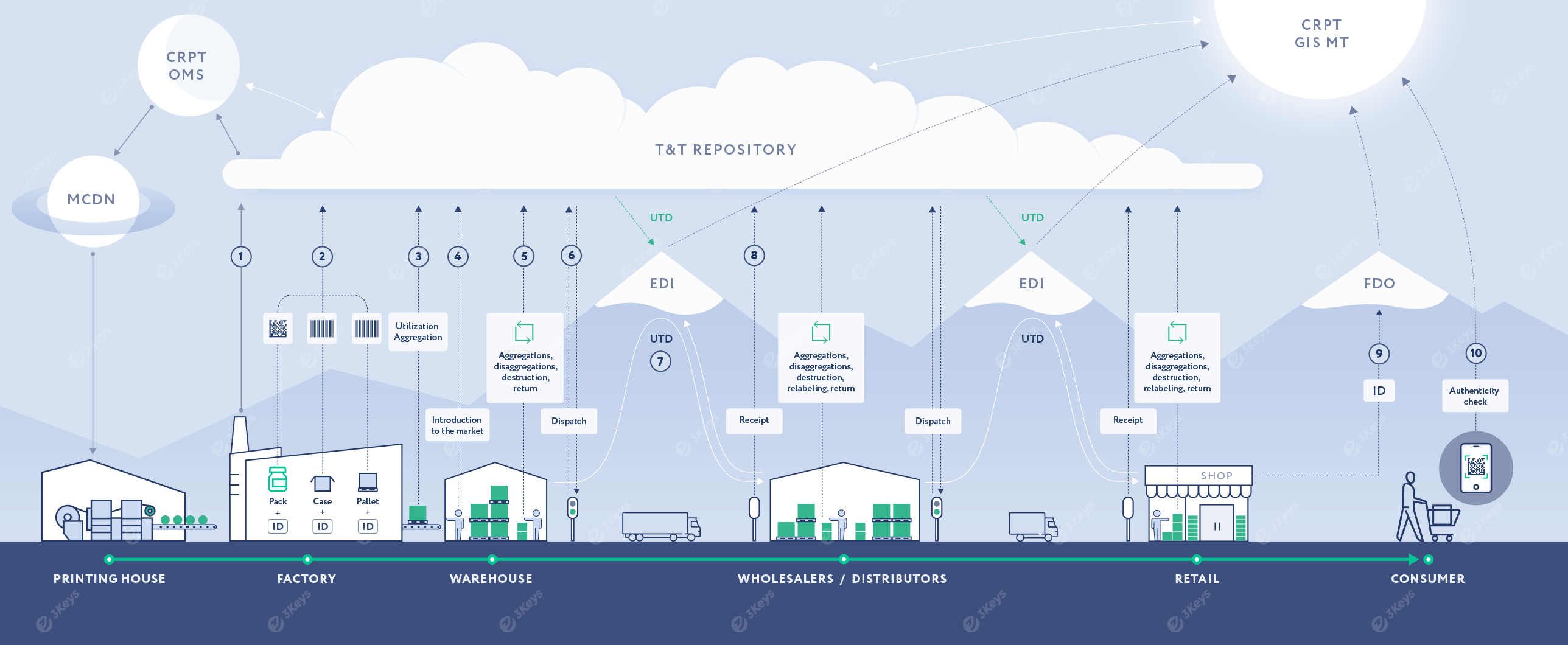
The manufacturer places an order for unique serial numbers with crypto codes for each produced trade item (SKU) based on the production plan. The serial numbers and crypto codes are emitted by the OMS CRPT system. The manufacturer can choose between direct labeling (and receive back Serial numbers from OMS) or specify a printing house as the service-provider, in which case the serial numbers will be retrieved directly by the printing house via the MCDN system and pre-printed as DataMatrix codes on the packaging material.
For direct labeling scenario, these serial numbers are encoded to Data Matrix codes and applied to individual consumer packages during production. Alternatively, packaging with pre-printed DataMatrix codes is used. This process makes the finished product serialized. The packages are placed into cases and other forms of containers - then further aggregated into pallets. Each level of a container is labeled with a unique SSCC of GS1-128 codes assigned by the manufacturer. Codes are associated in a parent/child structure.
Each applied code and the hierarchical structure is stored in the private repository. It is then further reported to OMS and GIS MT systems, along with additional attributes, such as expiry date.
After the end of the production of a batch and the quality control procedure, the serialized packages are reported to GIS MT as available for further sale.
At any given point in the supply chain, the product aggregation can change. When this happens it is reflected in the repository and reported to GIS MT. Likewise, products can be written-off for various reasons, such as damages, expiration, shortages, etc.
After the serialized goods are packed in cases and pallets, they are dispatched from the manufacturer to the next supply chain partner. Each serialized container is scanned and the unique codes are included in a Universal Transfer Document (UTD).
The UTD is sent to a certified EDI provider, which is then forwarded to the receiver of the shipment.
When receiving a serialized shipment, the wholesaler scans each serialized container to verify it against the inbound UTD. Any discrepancies are resolved by additional EDI documents, such as UCD. Once the UTD is electronically signed by the receiver, the change of ownership occurs. Lastly, the information is pushed to GIS MT.
Finally, when the serialized package is sold at a retail store to the end-consumer, the Data Matrix is scanned at the POS. The unique serial numbers are included in the electronic cash receipt, which is sent via a Fiscal Data Operator to GIS MT.
Any consumer can scan a DataMatrix code with a phone App to verify the authenticity of the product and learn more about its origin.
*To be updated after the approval of regulatory documents.
Proposed architecture of Russian Track & Trace regulations for dietary supplements import*
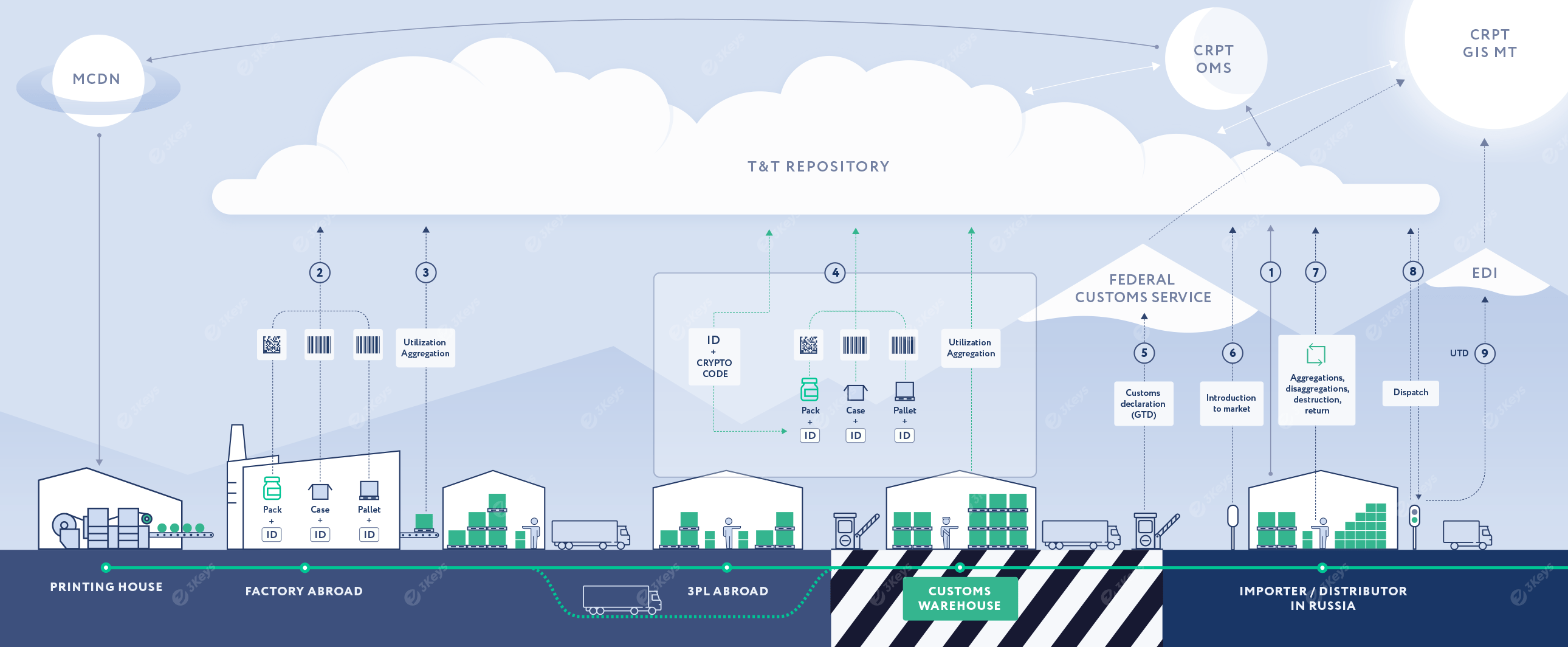
The importer places an order for unique serial numbers with crypto codes for each produced trade item (SKU) based on the production/purchase plan. The serial numbers and crypto codes are emitted by the OMS CRPT. The importer can choose between direct labeling (and receive back Serial numbers from OMS and pass them to the Labeling facility) or specify local or abroad printing house as the service-provider, in which case the serial numbers will be retrieved directly by the printing house via the MCDN system and pre-printed as DataMatrix codes on the packaging material.
For direct labeling scenario, these serial numbers are passed to the Labeling facility, where they are encoded to Data Matrix codes and applied to individual consumer packages during production. Alternatively, packaging with pre-printed DataMatrix codes is used. This process makes the finished product serialized. The packages are placed into cases and other forms of containers and further aggregated into pallets. Each level of the container is labeled with a unique SSCC of GS1-128 codes assigned by the manufacturer. Codes are associated in a parent/child structure.
Each applied code and the hierarchical structure are stored in the private repository and further reported by the importer to OMS and GIS MT systems. Other additional attributes, such as the expiry date, are also prevalent.
Alternatively, the serialization process can take place at a warehouse outside Russia or at the customs warehouse. The full hierarchy has to be reported to GIS MT before the shipment can be customs-cleared.
The importer, or the customs broker, submits a customs declaration (GTD) to the Federal Customs Service. The GTD includes a list of unique container codes. The Federal Customs Service verifies the codes with GIS MT and clears the shipment.
In order to make the products available for further sale, the importer reports the unique container codes to GIS MT.
At any given point in the supply chain, the product aggregation can change. When this happens, it is reflected in the repository and reported to GIS MT. Likewise, products can be written-off for various reasons, such as damages, expiration, shortages, etc.
After the serialized goods are packed in cases and pallets, they are dispatched from the importer to the next supply chain partner. Each serialized container is scanned and the unique codes are included in a Universal Transfer Document (UTD).
The UTD is sent to a certified EDI provider, which is then forwarded to the receiver of the shipment.
*To be updated after the approval of regulatory documents.