March 2017 – Serialization SSU – All Products must be labeled in compliance with Legislative Decree No. 41 of 2017.
February 2018 – Registration DENR – All local manufacturers and warehouses have to register their GLN’s in the DENR authority portal. Do not apply for overseeing suppliers.
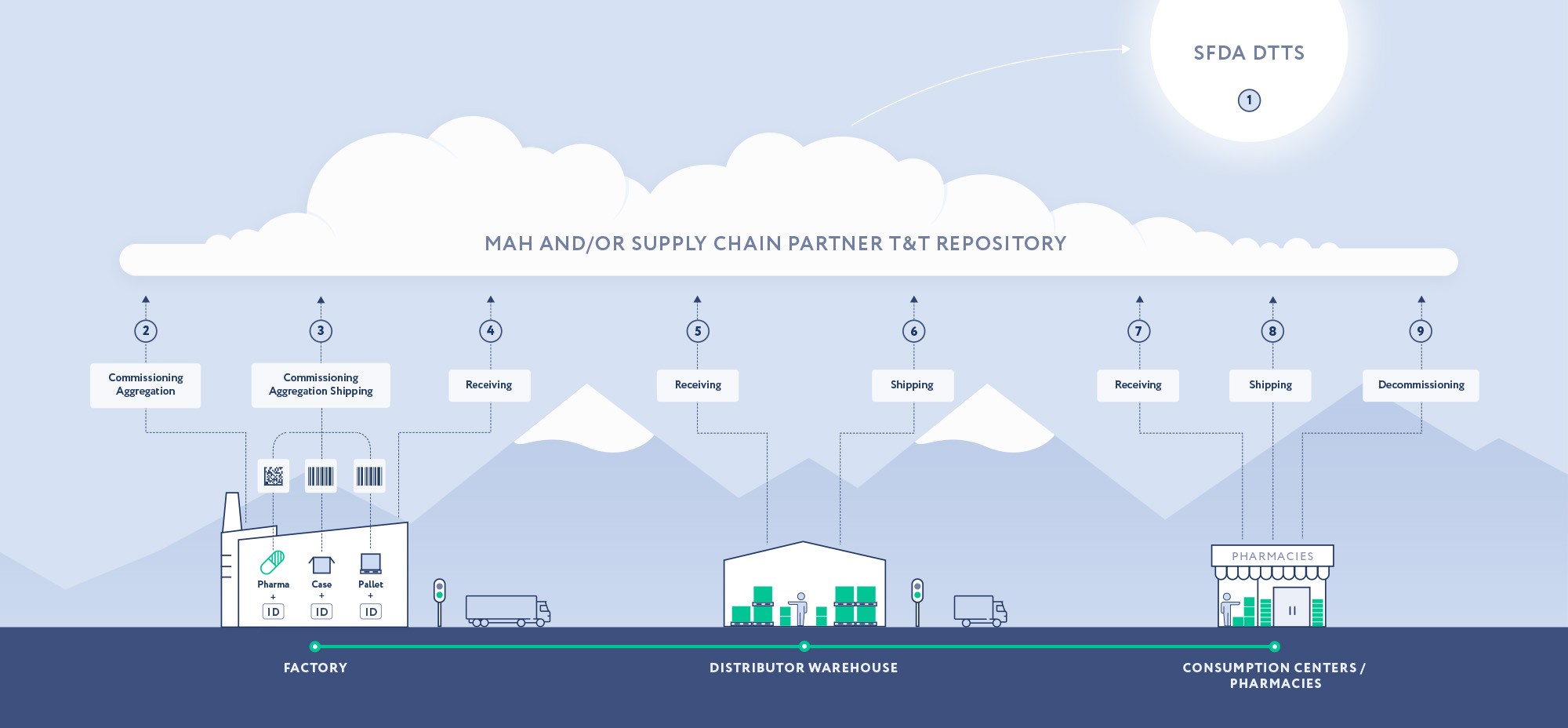
7 January 2019 – Reporting DTTS – Stakeholders start reporting SNs through the new authority platform called DTTS, Drug Track & Trace System.
August 2020 – Aggregation – All Companies representing Manufacturers/MAHs are required to be compliant with Aggregation reporting requirements.
Who is affected?
- Manufacturers
- Warehouses
- Pharmacies
- Medical and Dispense Centers
The external manufacturers (oversee suppliers with production outside the KSA) have the possibility to provide their agents with a flat list of SN.