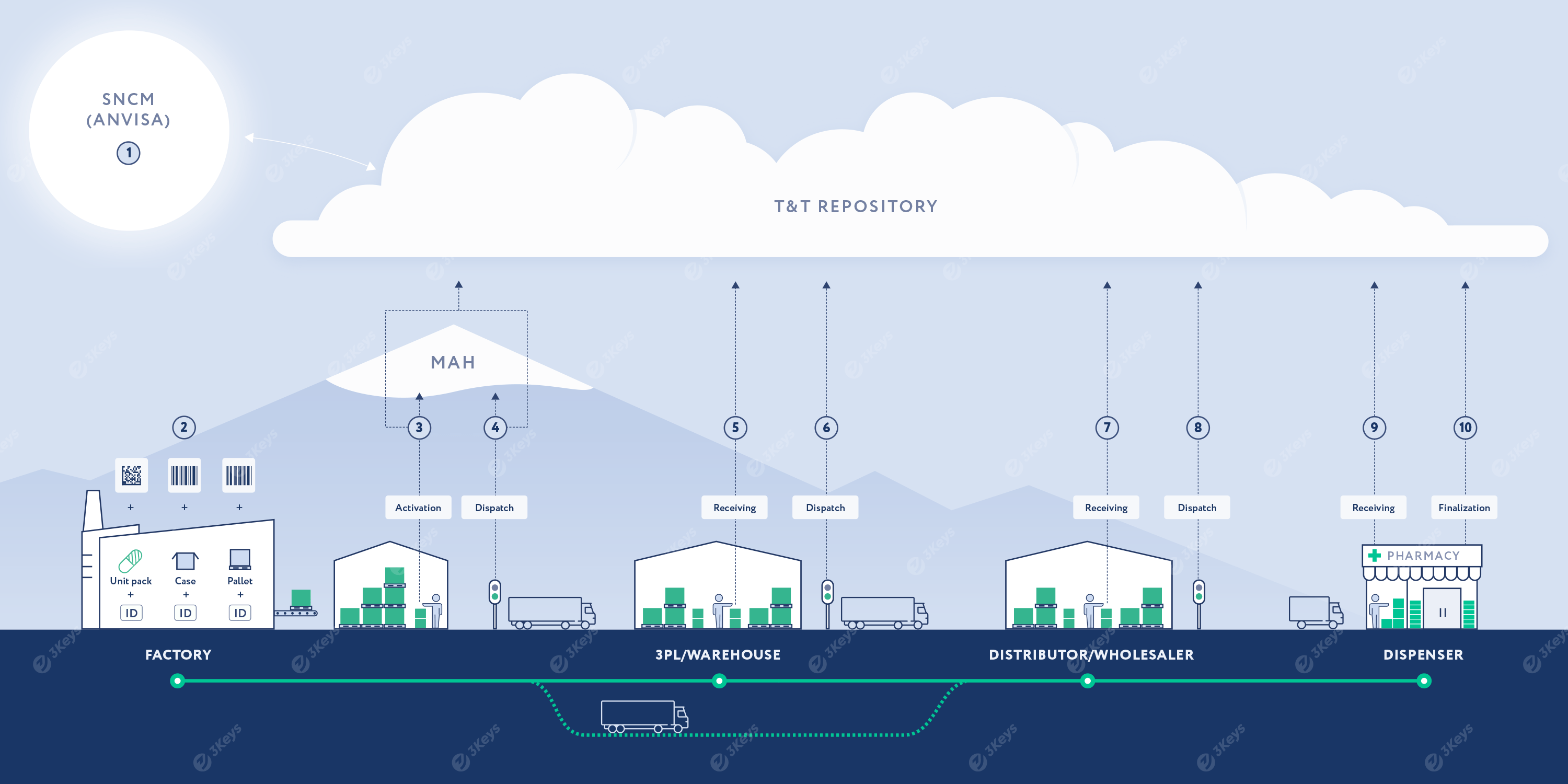
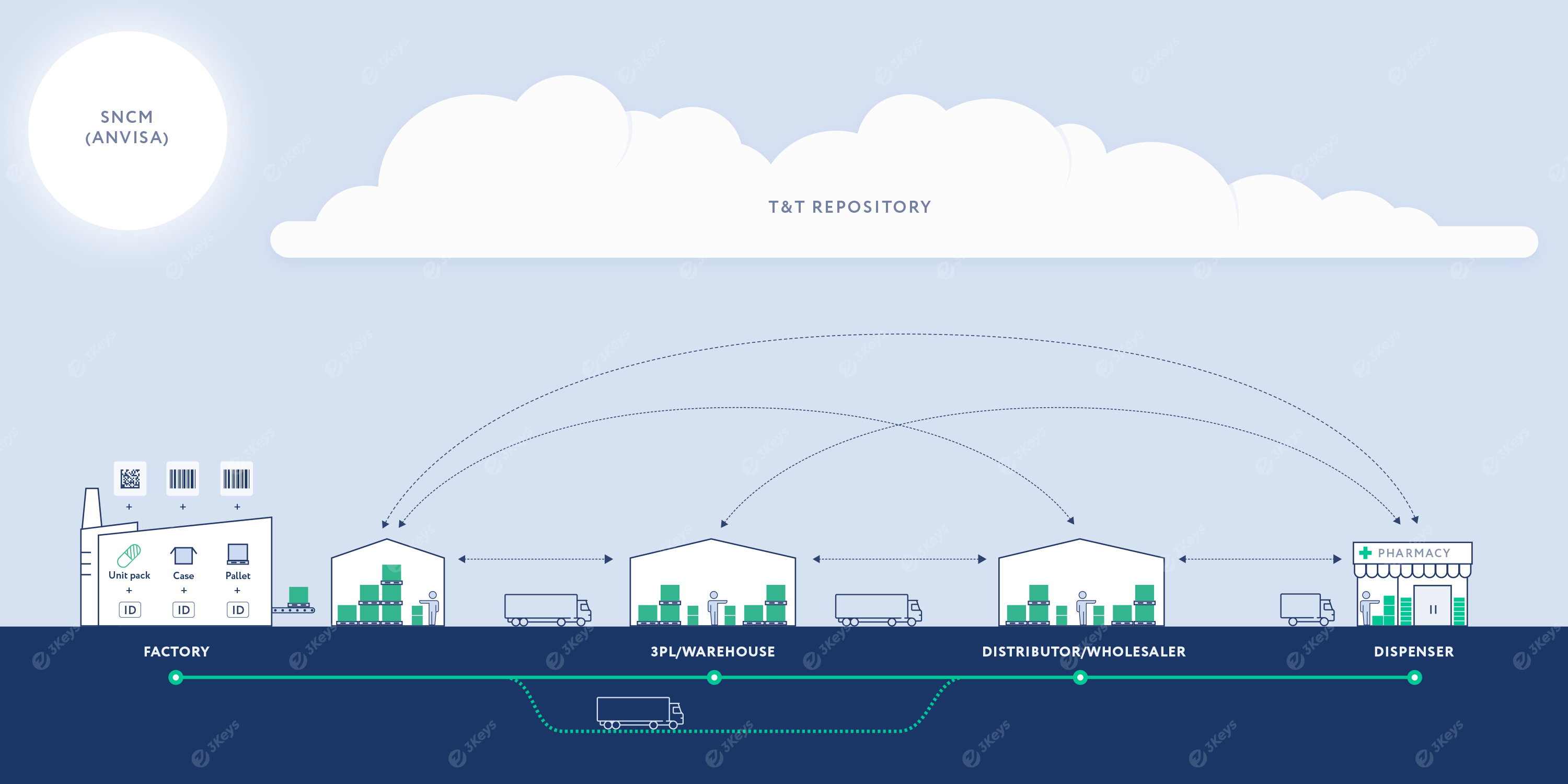
Highlights of the reporting deadlines to ANVISA (SNCM):
Up to October 2021: 5% of the released batches in the Brazilian market should be serialised and reported.
November 2021–April 2022: 10% of the released batches in the Brazilian market should be serialised and reported.
1) Local Manufacturing
Manufacturers should have the following minimum percentages of packaging lines serialised and events reported as follows:
I—30% as of April 2022
II—70% as of April 2023 and
III—100% as of April 2024
2) Imports
Importing companies must have the following minimum percentages of batches marketed as serialised and reported:
I—30% as of April 2022
II—70% as of April 2023 and
III—100% as of April 2024
3) Distributors, Wholesalers, and Dispensers
April 2024: Distributors and wholesalers must report all events related to serialised drugs traded from April 2024 onwards.
III – 100% as of April 2024