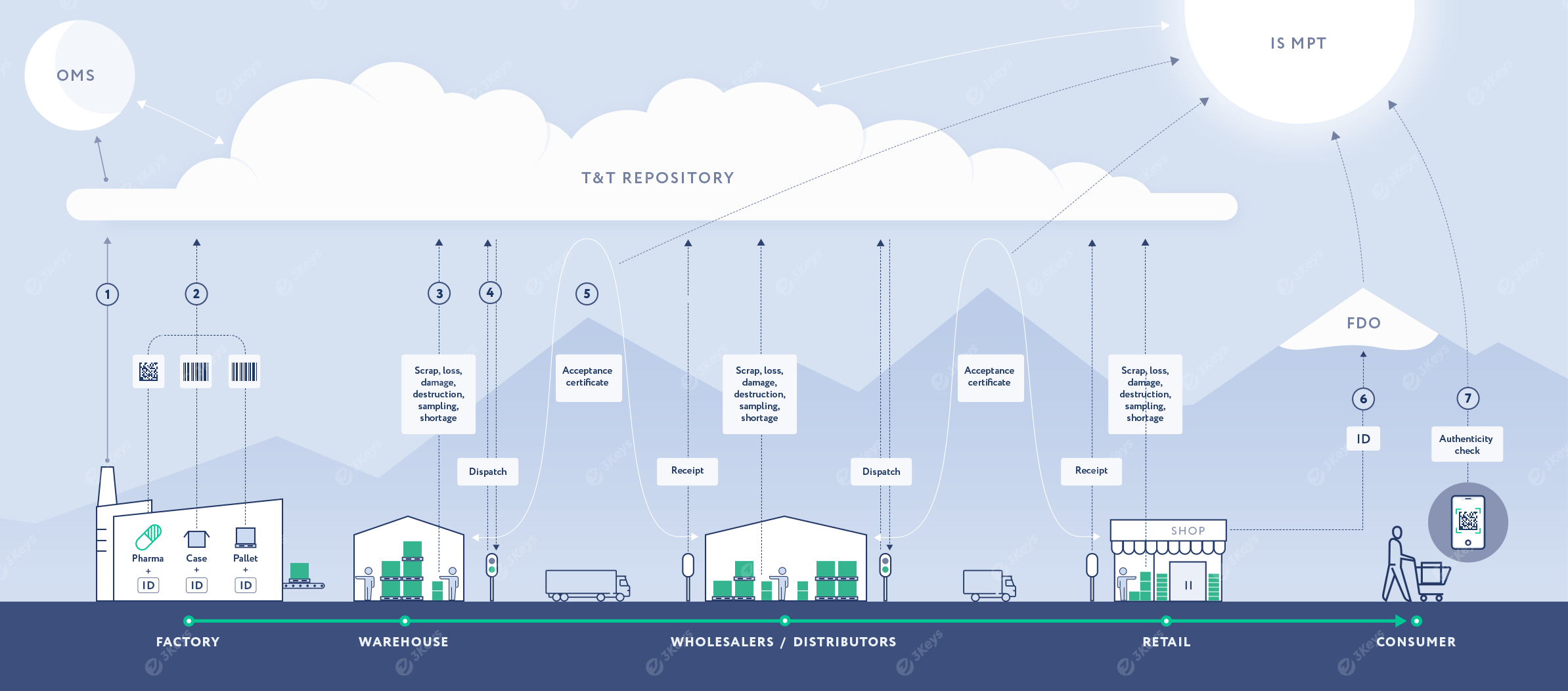
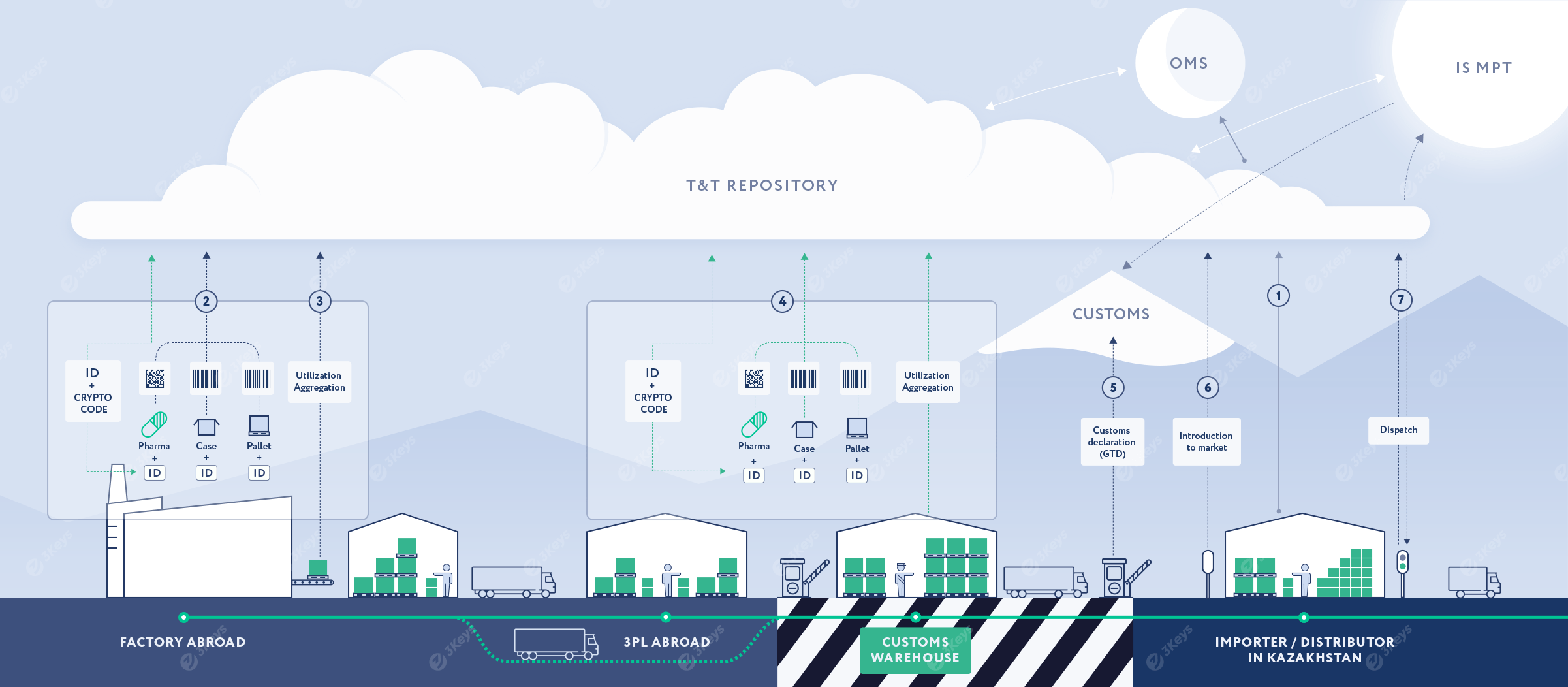
September 1, 2019 – July 31, 2021 the experiment on labeling of medicines lasted.
Labeling is introduced for medicines manufactured from July 1, 2022 (90 items). The first stage – serialization.
Proposed Timeframe:
- 1 July 2022 –Introduction of labelling for approved 93 of medicines (1% from all drugs)
- 1 October 2022 -expansion list of drugs at least for 20%
- 1 January 2023 -expansion of the list of drugs at least for 60% and starting traceability of marked drugs
- 1 April 2023 -expansion list of drugs at least for 80%
- 1 July 2023 -Implementation 100% marking and traceability
The most current information about the timing of mandatory labeling, legislative acts, technical documentation, as well as the beginning of the experiment of new product groups you can find on the websites of our partners: Naqty Onim and QazMarka.