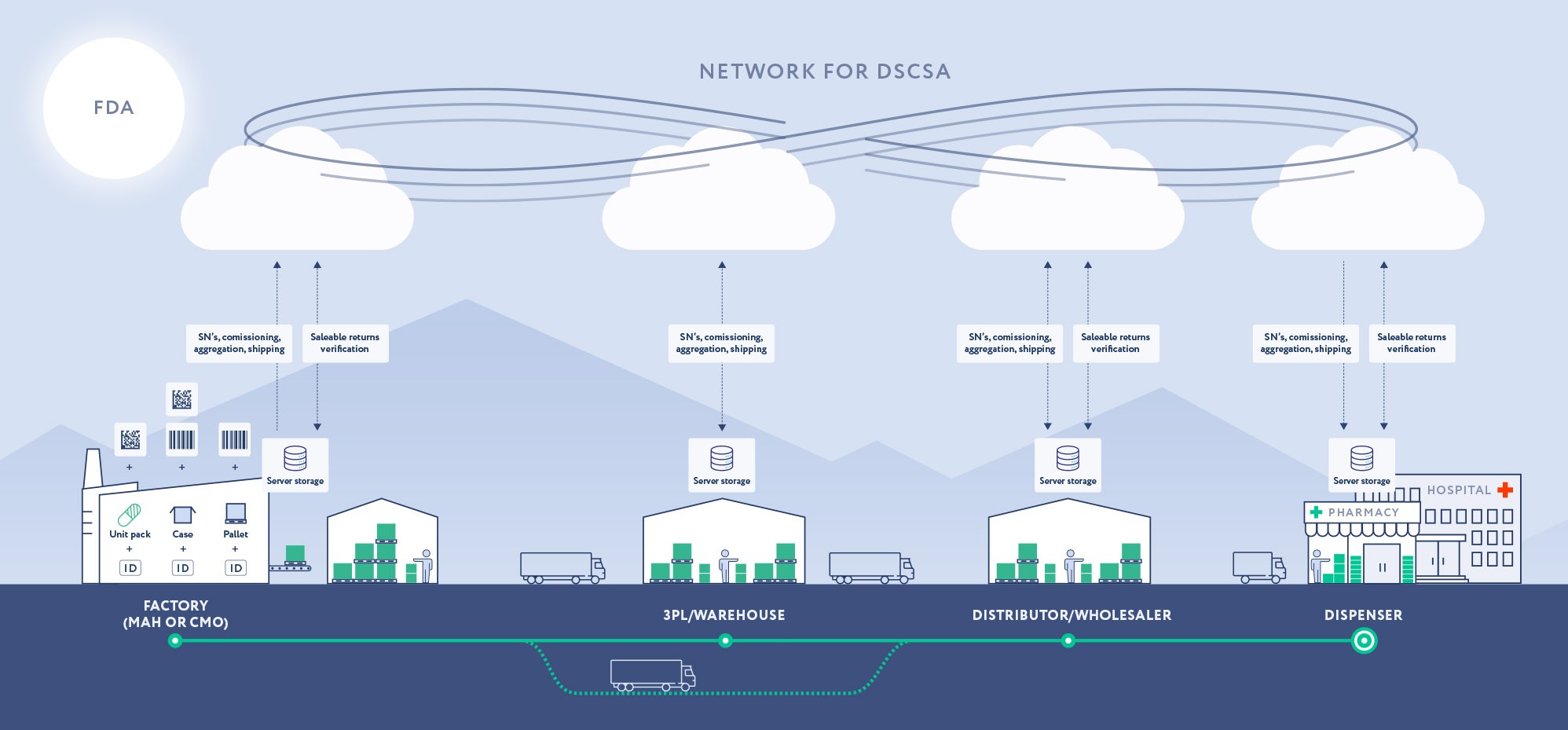
To achieve traceability of certain prescription drugs, the Drug Supply Chain Security Act (DSCSA) was signed on November 27, 2013. superseding various state regulations. Each prescription drug product must have a unique product identifier encoded in a 2D barcode that contains the products National Drug Code (NDC), serial number, lot number, expiration date, and can be used to track and trace the product throughout the supply chain. No national repository for reporting and storing serialization data is provided for the US market. Manufacturers, wholesale distributors, repackagers, and dispensers must keep a record of the transaction history for each prescription drug product, including the product’s unique identifier, transaction information, and the information about the previous and next owner of the product.
Wholesale distributors and dispensers must verify the authenticity of the prescription drug before dispensing it to the patient, and all stakeholders in the supply chain must investigate and report any suspected products. Saleable returns are verified by wholesalers.
Serialization information is exchanged at a lot level starting from Nov 2015 and serialization indicators must be printed on prescription drugs from Nov 2017.
On November 27. 2023 manufacturers start to provide full aggregation to downstream partners.
Choosing and implementing the right traceability solution will be the key to complying with strict regulations and building effective data exchange with your business partners.