1
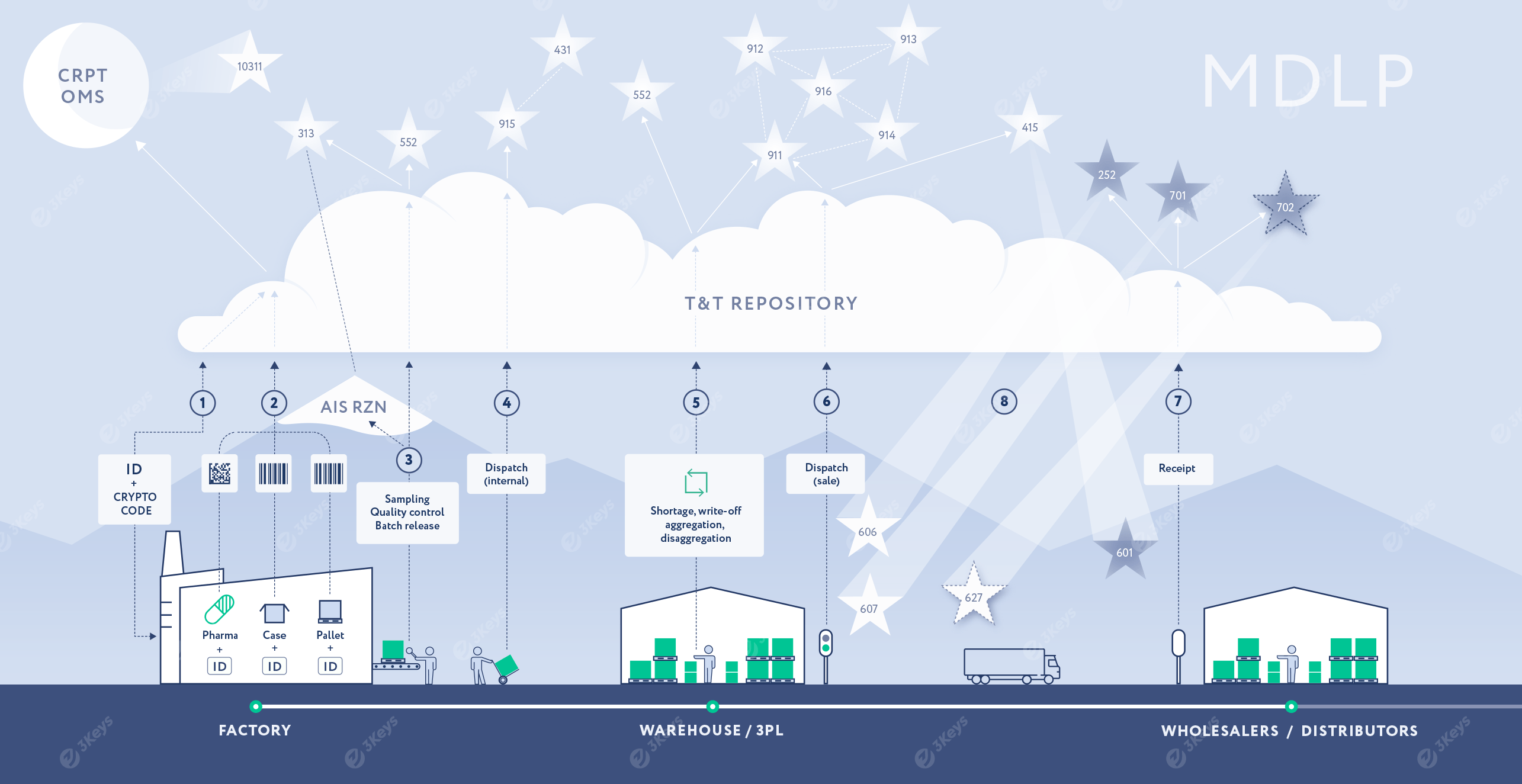
The product MAH generates serial numbers based on the production plan, and it orders and retrieves crypto codes from the CRPT OMS system. The serial numbers are passed to the production site (own production or CMO).
2
The serial numbers and crypto codes are encoded as GS1 Data Matrixes and applied to the individual end-consumer packages. The packages are aggregated to cases and then to pallets, each of them labelled with a unique SSCC bar code. Once the production order is closed (or after batch release), the full hierarchy of codes is passed to the T&T repository. The list of serialised consumer packages is reported back to OMS along with the batch and expiration date information. The OMS system sends a 10319 message with the same content to the MDLP system.
3
During the quality control process (as well as during further logistics processes), certain packages can be identified as not being permitted for further sales and are reported to the MDLP system via the 552 "withdrawal" message.
4
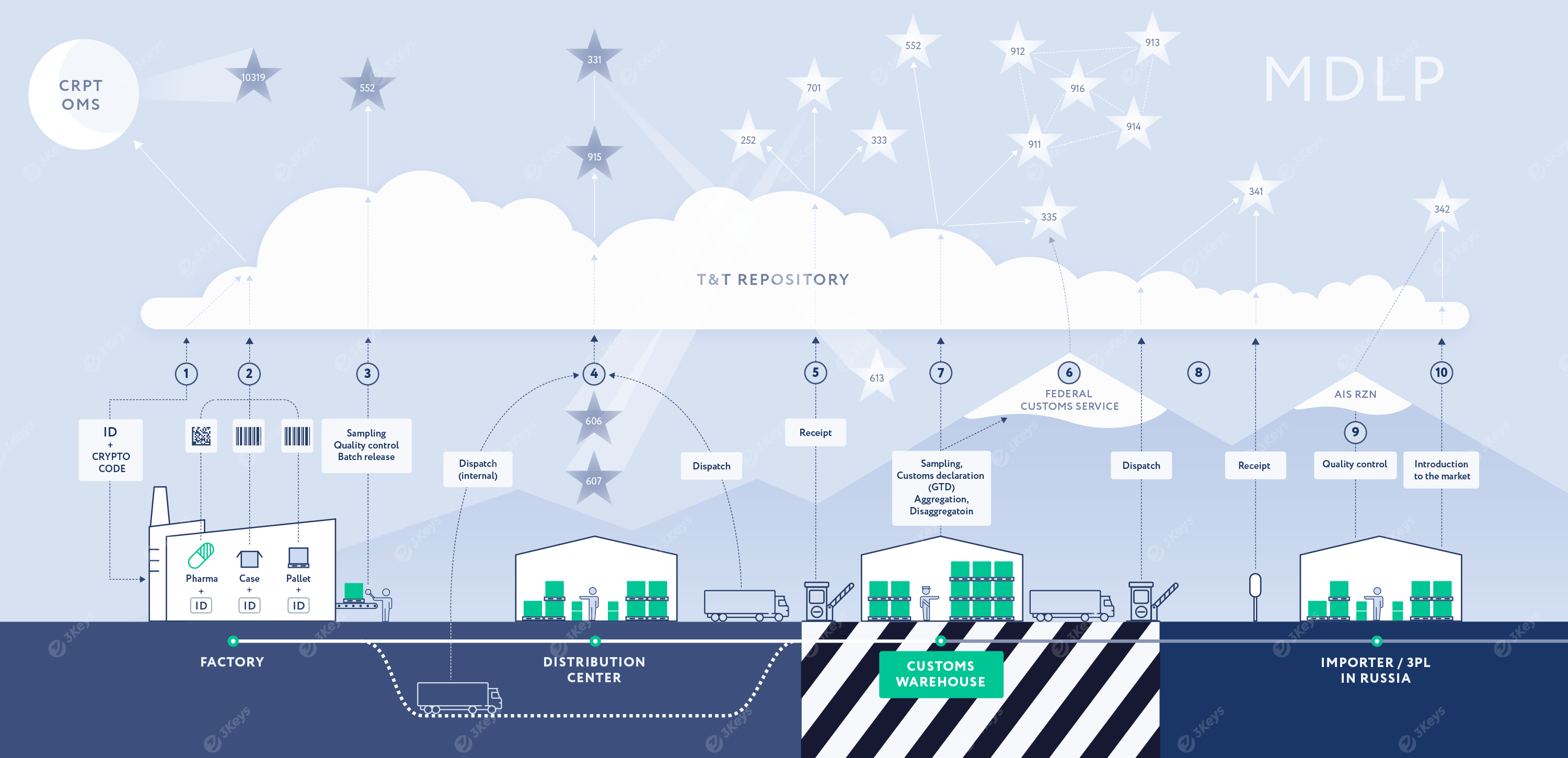
**During the dispatch process, when the serialised pallets, cases, or individual packages are loaded and shipped to Russia—either from the production facility or a distribution centre—each top-level serial number is scanned and reported to the T&T repository. The full hierarchy is reported to MDLP by the MAH via the 915* "multi-pack" message, followed by a 331 "foreign shipment" message, which specifies the Russian importer as the receiving party. The importer is notified of the shipment via MDLP by the 613 "Foreign shipment notification". If needed, the receiver can retrieve the full hierarchy of serial numbers packed inside the reported SSCCs from MDLP.
5
**Upon receiving the shipment at the customs warehouse, the importer scans the items and compares the serial numbers with the ones received via the 613 notification. The importer confirms the shipment via a 701 "accept" notification or rejects all or part of the serial numbers via a 252 "Refusal receiver" message.
6
The importer or the customs broker submits a customs declaration (GTD) to the Federal Customs Service. The GTD includes a list of unique container codes. The Federal Customs Service verifies the codes with MDLP and clears the shipment.
7
The importer reports the 335 "FTS data" to MDLP with a list of cleared SSCCs and the GTD number. MDLP verifies the information with the Federal Customs Service. If any serialised items have been sampled at the customs warehouse, these items are reported to MDLP via the 552 "withdrawal" message. The appropriate combination of 91x messages is used to reflect the hierarchy changes in MDLP.
8
The products are moved from the customs warehouse to the importer's warehouse or 3PL location. This movement is reported to MDLP via a 341 "Receive importer" report.
9
Quality control certifies that the imported batch can be released to the market and enters the data into the AIS RZN system.
10
The top-level serialised containers are reported to MDLP as being introduced into circulation via the 342 "Release in circulation" message, referencing the document number re-exported to AIS RZN. The MDLP verifies the data with AIS RZN, and the goods are made available for further sale within the local supply chain.
* 915 can also be reported at any earlier stage, in which case any further aggregation changes need to be reported separately.
**(4) and (5) describe the “direct acceptance” process. The “reverse acceptance” process is also available, in which case the receiver (importer) replies with the 332 “foreign import message”, and the product MAH confirms the shipment via the 701 “accept” message. If the importer and the MAH are the same legal entity, a single 333 report is sent to MDLP upon importing the goods instead of the message sequence described in (4) and (5).